SCIENCE
CerFlux science advances human-relevant New Approach Methodologies (NAMs) that combine biomimetic engineering, microphysiological systems, and high-throughput ex vivo and in silico (computational and AI/ML) tumor models to better understand how individual tumors respond to therapy before treatment begins.
This page highlights peer-reviewed publications, high-impact conference abstracts, issued patents, and funded research that underpin platforms such as POET, BEST, SMART, and Lab-on-a-Brane technologies. Together, these efforts reflect a commitment to building human-relevant technologies that support more informed, patient-specific decision-making across translational cancer research and development.
Evaluation of a Novel Pan-RAS Inhibitor in 3D Bioprinted Tumor Models
De Nobrega, D; Eiler, LC; Ahirwar, P; Nallapu, S; Rawal, UP; Crawford, CL; Buchsbaum, DJ; Keeton, A; Maxuitenko, YY; Chen, X; Piazza, GA; Tsung, A; Budhwani, KI.
Publication | Cancers | 2025 | DOI: 10.3390/cancers17182958

Bioprinted 3D tumor models are an innovative approach that replicates the structure and environment of real tumors, offering an alternative to animal models for testing new drugs. In this study, we employ these models to evaluate a novel inhibitor targeting RAS proteins, common drivers of many cancers. By recreating the complex architecture of tumors in the laboratory, we demonstrate that this compound selectively eliminates tumor cells harboring RAS mutations while sparing cells without these mutations. Our work highlights the promise of 3D bioprinted tumor models for guiding drug development and advancing treatment strategies for cancers driven by RAS alterations.
Keywords: new approach methods; drug discovery; 3D bioprinting; colorectal cancer; RAS mutations; pan-RAS inhibitors; ex vivo tumor models
The Scienthetic Method: From Aristotle to AI and the Future of Medicine
Budhwani, KI.
Publication | British Journal of Cancer | 2024 | DOI: 10.1038/s41416-024-02841-1

While AI holds immense potential for accelerating advances in oncology, we must be intentional in developing and applying these technologies responsibly, equitably, and ethically. One path forward is for cancer care providers and researchers to be among the architects of AI and its adoption in medicine. Given the limitations of traditional top-down, hypothesis-driven design in an exponentially expanding data universe, on one hand, and the danger of spiraling into artificial ignorance (ai) from rushing into a purely ‘synthetic’ method on the other, this article proposes a ‘scienthetic’ method that synergizes AI with human wisdom. Tracing philosophical underpinnings of the scientific method from Socrates, Plato, and Aristotle to the present, it examines the critical juncture at which AI stands to either augment or undermine new knowledge. The scienthetic method seeks to harness the power and capabilities of AI responsibly, equitably, and ethically to transcend the limitations of both the traditional scientific method and purely synthetic methods, by intentionally weaving machine intelligence together with human wisdom.
Efficacy assessment of a novel pan-RAS inhibitor in KRAS-mutant and wild type colorectal 3D bioprinted organoid tumor tissue
Ahirwar, P; Charania, AA; Zuaiter, DR; Eiler, LC; Nizamuddin, A; Crawford, CL; Maxuitenko, YY; Piazza, GA; Budhwani, KI.
Abstract | Journal of Clinical Oncology | 2024 | DOI: 10.1200/JCO.2024.42.23_suppl.91
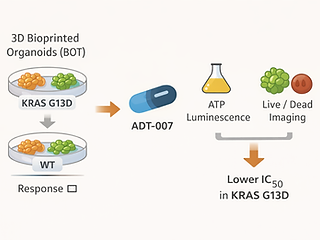
This ASCO meeting abstract evaluates ADT-007, a novel pan-RAS inhibitor, using a high-throughput ex vivo platform with 3D bioprinted organoid tumor (BOT) tissue models of colorectal cancer. The team generated BOTs from a KRAS G13D–mutant CRC cell line (HCT116) and a wild-type CRC line (HT29) and measured drug response via ATP luminescence plus high-content live/dead imaging. In these BOT models, ADT-007 showed a lower IC₅₀ in KRAS-mutant tissue than in wild-type tissue, consistent with separate in vitro/in vivo observations. The authors conclude that broadly acting pan-RAS inhibition, paired with BOT-based testing that better captures tumor microenvironment features, may help assess efficacy across RAS-driven cancers beyond a single KRAS allele.
Assessment of KRAS G12C inhibitors for colorectal cancer
Piazza, GA; Chandrasekaran, P; Maxuitenko, YY; Budhwani, KI.
Publication | Frontiers in Oncology | 2024 | DOI: 10.3389/fonc.2024.1412435

Colorectal cancer (CRC) is a common and often lethal cancer worldwide, and roughly 45% of CRC tumors carry activating KRAS mutations. KRAS is the most frequently mutated oncogene in humans (implicated in ~25% of all cancers), and these mutations can lock KRAS “on,” driving MAPK/AKT signaling that promotes uncontrolled growth, survival, and other features of malignancy. Although KRAS was long viewed as undruggable, the FDA has now approved two direct KRAS inhibitors—sotorasib and adagrasib—that covalently inactivate KRAS^G12C. These agents have shown meaningful benefit in KRAS^G12C-mutant NSCLC, but they’ve been far less effective in CRC, for reasons that remain incompletely understood. Because similar limitations may affect other mutant-specific KRAS inhibitors in development, it’s critical to understand the biologic basis of resistance. This review summarizes clinical trial results of KRAS^G12C inhibitors in CRC (as monotherapy and in combinations), outlines mechanisms that drive resistance, and discusses emerging RAS-targeting strategies designed to overcome or bypass these resistance pathways.
A comprehensive preanalytical protocol for fresh solid tumor biospecimens
Charania, AA; Pokal, AG; Zuaiter, DR; Crawford, CL; Esnakula, AK; Islam, M; Kim, AC; Budhwani, KI.
Publication | Methods | 2024 | DOI: 10.1016/j.ymeth.2024.06.005

Nearly seventy percent of diagnostic lab test errors occur due to variability in preanalytical factors. These are the parameters involved with all aspects of tissue processing, starting from the time tissue is collected from the patient in the operating room, until it is received and tested in the laboratory. While there are several protocols for transporting fixed tissue, organs, and liquid biopsies, such protocols are lacking for transport and handling of live solid tumor tissue specimens. There is a critical need to establish preanalytical protocols to reduce variability in biospecimen integrity and improve diagnostics for personalized medicine. Here, we provide a comprehensive protocol for the standard collection, handling, packaging, cold-chain logistics, and receipt of solid tumortissue biospecimens to preserve tissue viability.
Characterizing differential efficacy and phenotypic response to proteasome and survivin inhibitors in colorectal cancers using a high throughput organoid assay
Zuaiter, DR; Ahirwar, P; Pokal, AG; Patel, ZH; Charania, AA; Crawford, CL; Sewell-Loftin, MK; Tsung, A; Kim, A; Budhwani, KI.
Abstract | Journal of Clinical Oncology | 2024 | DOI: 10.1200/JCO.2024.42.3_suppl.153

This ASCO GI 2024 abstract describes a high-throughput, ex vivo “bioprinted organoid tumor” (BOT) platform designed to capture drug responses in 3D colorectal cancer tissue models beyond what standard monolayer and xenograft assays can predict. Using HT-29 colorectal adenocarcinoma cells embedded in a printable bioink, the team printed BOTs in different geometries and treated them with the proteasome inhibitor bortezomib and the survivin inhibitor YM-155, then assessed response via live/dead immunofluorescence and observed morphologic/phenotypic changes. Both agents produced a dose-dependent response, and importantly, the BOTs showed disrupted self-assembly/phenotypic modulation at and even below “effective” doses; signals that ATP-only assays can miss and that could lead to overstated efficacy when control wells proliferate differently. The authors conclude that functional, high-throughput ex vivo drug-response prediction platforms like BOTs could improve preclinical screening by capturing phenotype alongside viability.
SMART Microchamber Array for Multiplexed ex vivo Drug Screening
Budhwani, KI.; Budhwani, BK; Budhwani, KK.
Issued Patent | USPTO | US11833514B2 | Filed 2021 | Approved 2023

This issued patent strengthens the scientific and engineering foundation of Simple Microchamber Array Technology (SMART) by expanding the foundation for multiplexed, parallel exposure of intact biological samples to many test conditions in a single run. Building on SMART’s biomimetic microchamber array architecture, this patent further supports controlled, organized delivery of multiple fluids across defined regions of a single tissue specimen, enabling efficient side-by-side evaluation while preserving native tissue structure. The result is a practical path to higher-throughput, tissue-sparing workflows that can accelerate comparative compound assessment and iteration in human-relevant ex vivo testing, especially when patient material is limited.
Predictive efficacy biomarker for chemotherapy agents against triple-negative breast cancer bioprinted organoid tumors (BOTs) using solid tumor biopsy-on-a-chip
Bollenbecker, SE; Patel, ZH; Punjani, Z; Charania, AA; Patel, HK; Abott A; Kunkle, K; Sewell-Loftin, MK; Grossman, G; Budhwani, KI.
Abstract | Cancer Research | 2023 | DOI: 10.1158/1538-7445.SABCS22-P6-01-38

This SABCS 2022 / Cancer Research supplement abstract (P6-01-38) presents a way to expand triple-negative breast cancer (TNBC) tissue into 3D bioprinted organoid tumors (BOTs) that mimic core biopsies for ex vivo chemotherapy sensitivity/resistance testing. The team bioprinted MDA-MB-231 TNBC cells in an alginate-based bioink into biopsy-like geometries, cured/crosslinked the constructs, and then loaded them into a solid tumor “biopsy-on-a-chip” platform for drug exposure with live/dead immunofluorescence readouts. They report an optimized crosslinking/geometry workflow compatible with the chip and demonstrate diffusion of small molecules into the bioprinted tissue to substantial depth, supporting multi-agent testing. Overall, the work argues that BOT-based ex vivo platforms could provide more clinically relevant efficacy signals than conventional in vitro models and help guide personalized chemotherapy strategies.
Simultaneous rapid preclinical therapeutic evaluation in a novel ex vivo bioreactor
Bollenbecker, SE; Patel, ZH; Punjani, Z; Charania, AA; Patel, HK; Saleh, MN; Budhwani, KI.
Abstract | ESMO Open | 2022 | DOI: 10.1016/j.esmoop.2022.100729

This abstract argues that preclinical drug testing is often poor at predicting human outcomes because standard static cultures and many animal models don’t capture the human tumor microenvironment, contributing to high clinical failure rates. Here we present a microfluidic ex vivo tissue model built with engineered microporous membranes (soft lithography and electrospinning) and multicellular healthy and tumorous microtissues using pancreatic and colorectal cell lines. Permeability testing showed selective transport supporting barrier/transport function. When “tumorous” tissue was connected upstream of “healthy” vascular and muscle tissue, we observed migration and invasion, and identified invasive subpopulations with more aggressive proliferation than cells that stayed at the original tumor site. Overall, we propose human-relevant, ex vivo models as a path toward faster, more comprehensive pharmacologic testing and more predictive, personalized treatment guidance.
